
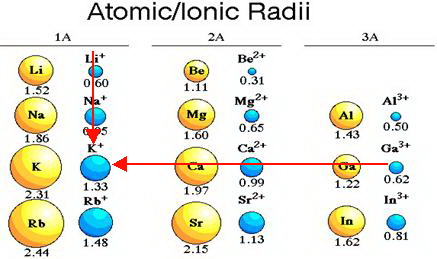
The general trend is that radii increase down a group and decrease across a period. (b) Covalent radii of the elements are shown to scale. The atomic radius for the halogens increases down the group as n increases. (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. Covalent Radii of the Halogen Group Elementsįigure 1. The trends for the entire periodic table can be seen in Figure 1. This trend is illustrated for the covalent radii of the halogens in Table 5 and Figure 1. Consequently, the size of the atom (and its covalent radius) must increase as we increase the distance of the outermost electrons from the nucleus. Thus, the electrons are being added to a region of space that is increasingly distant from the nucleus. We know that as we scan down a group, the principal quantum number, n, increases by one for each element. We will use the covalent radius ( Figure 1), which is defined as one-half the distance between the nuclei of two identical atoms when they are joined by a covalent bond (this measurement is possible because atoms within molecules still retain much of their atomic identity). However, there are several practical ways to define the radius of atoms and, thus, to determine their relative sizes that give roughly similar values. The quantum mechanical picture makes it difficult to establish a definite size of an atom. With just a few clicks, you can create three-dimensional versions of the periodic table showing atomic size or graphs of ionization energies from all measured elements.
They are (1) size (radius) of atoms and ions, (2) ionization energies, and (3) electron affinities.Įxplore visualizations of the periodic trends discussed in this section (and many more trends). These properties vary periodically as the electronic structure of the elements changes. An understanding of the electronic structure of the elements allows us to examine some of the properties that govern their chemical behavior. As we go down the elements in a group, the number of electrons in the valence shell remains constant, but the principal quantum number increases by one each time. Oxygen, at the top of group 16 (6A), is a colorless gas in the middle of the group, selenium is a semiconducting solid and, toward the bottom, polonium is a silver-grey solid that conducts electricity.Īs we go across a period from left to right, we add a proton to the nucleus and an electron to the valence shell with each successive element. For example, as we move down a group, the metallic character of the atoms increases. However, there are also other patterns in chemical properties on the periodic table. This similarity occurs because the members of a group have the same number and distribution of electrons in their valence shells. The elements in groups (vertical columns) of the periodic table exhibit similar chemical behavior. Describe and explain the observed trends in atomic size, ionization energy, and electron affinity of the elements.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
